Cell Therapy
Manufacturing
We offer end-to-end services, including cell culture and cell modification in process development and cGMP environments
End-to-End Cell Therapy Development and Manufacturing
We develop and manufacture diverse cell therapies, using numerous technologies, ranging from closed to open systems at different scales depending on your specific needs.
Autologous and Allogeneic Methods
Using the latest autologous and allogeneic methods, we help your products meet the exact specifications for patients in a clinical or commercial setting. We also have flexible and cost-effective services for process development and pre-clinical needs.

Cell Therapy Global Network
Our teams of experts provide the quality systems background and regulatory experience to help you navigate the important cell therapy product milestones, at any stage of the journey.

Pre-Clinical Through Commercial
We offer industry-leading expertise in process development and internalizing client cell therapy processes, including customization, development, qualification and validation activities for manufacturing and analytics. AGC Biologics’ services support every stage of the product journey, from pre-clinical through commercialization.
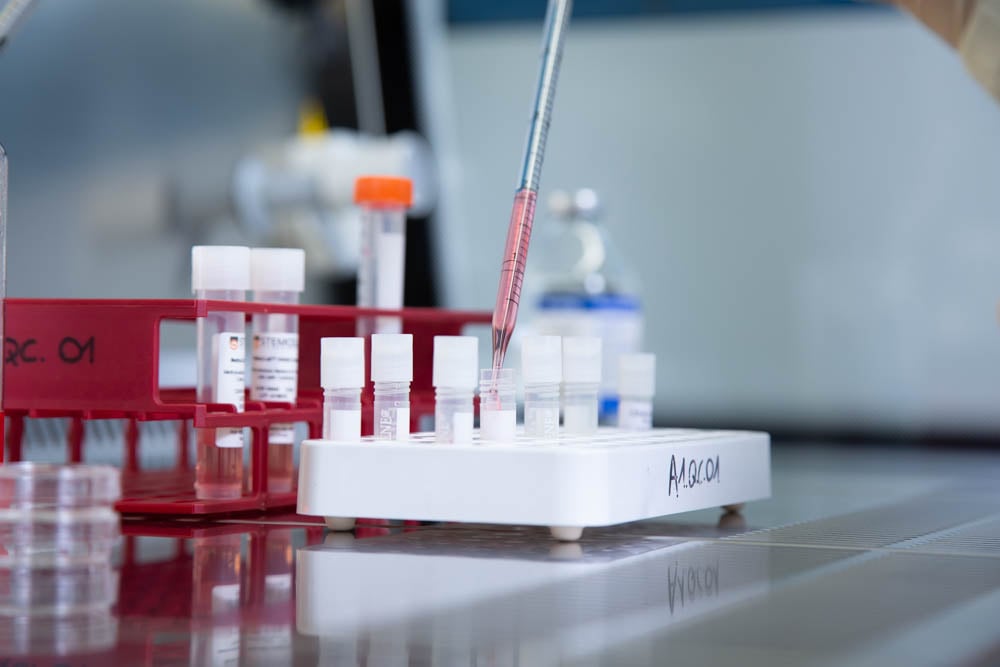
Best-in-Class Cell Therapy CDMO services
Offering top-tier cell therapy CDMO services, we excel in every stage of the life cycle, providing comprehensive support from initial development to clinical and commercial manufacturing. We specialize in cell culture and modification. Our capabilities encompass the creation of engineered cells at various scales, backed by analytical method development and qualification.
With a repertoire of over 160 in-house analytical tests thoughtfully designed for clinical and commercial supply, we stand as a comprehensive solution for cell therapy CDMO services.
Have a project in mind?
Cell Therapy Manufacturing Track Record
+0
Cell and gene therapy treated patients
0
Commercial cell therapy approvals
+0
Cell and gene therapy trials supplied in EU and US
Listen to Expert Interviews from Our Cell & Gene Center of Excellence in Milan

Click and drag video thumbnails to see more content.
Outsourcing strategies for allogeneic cell therapy
Learn about strategies for speeding up and optimizing the manufacturing of allogeneic cell therapies.
Creating cost-effective cell therapy programs with NK Cells
As this cell type continues to grow in popularity there are several strategies to consider for building a treatment program.
DownloadFill out the form to access "Opportunities to Accelerate Allogeneic Cell Therapy"
Fill out the form to access "The promise of NK cells and 3 strategies for creating a cost-effective program"
Full Lifecycle Cell Therapy Services

Cell Media
Strategic partnership with exosome cell manufacturing company RoosterBio to provide the best cell media possible.
We work with virtually any cell type, including Hematopoietic stem cells (HSCs), Human Mesenchymal Stem Cells (hMSCs), T Cells, NK Cells, and more.
95% of analytical tests performed in-house, reducing overall turnaround time and shortening your project timeline.








