
Adeno-Associated Vector Platform
A templated approach created by scientists with 30 years of experience.
Take the next stepEnd-to-end AAV services
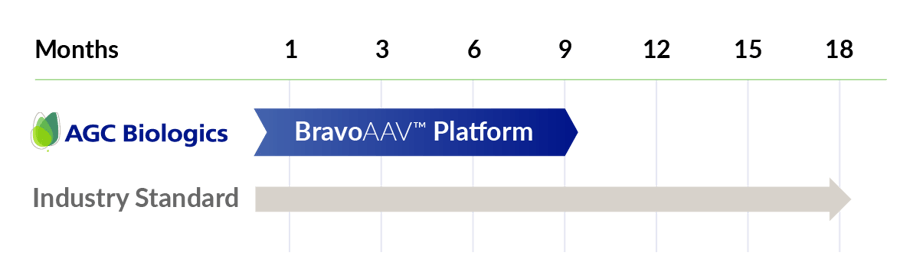
BravoAAV halves your time to GMP
Achieve your GMP goals in 9 months, accelerating your timeline from gene to the clinic!

Analysis on the promise of plug-and-play vector platforms
Explore how you can cut your timeline to cGMP in half using proven, templated processes.
Access ResearchBravoAAV fact sheet
Learn more about our capabilities with our adeno-associated virus platform.
View Fact SheetFill out the form to download this article
Product yields & network performance
BravoAAV offers both affordable costs on your first batch and ideal yields you need at any stage. We offer industry leading titers across different serotypes.
The platform is backed by a network of available capacity, high-quality materials and supplies.
Quality
GMP quality is at the center of everything we do. From pre-clinical through commercial, AGC Biologics has the knowledge and global regulatory expertise to help you meet your goals. Our sites in Milan and Longmont have regulatory and scientific expertise to navigate your product through each important stage, pre-clinical through commercial, as well as validated equipment and technology to support each key phase.

Peace of mind
We ease your path to commercialization through a new one-platform-fits-all approach. Give us your gene of interest, and let our team of 500+ highly experienced cell & gene professionals take care of the rest.
When & where you need us
We pride ourselves on being "Glocal." That means we are global and can serve you in your local market. With sites in North America and Europe, our viral vector services can meet your need anywhere in the world.


