Viral Vector
Manufacturing
We develop and manufacture viral vectors that meet clinical and commercial demands for gene therapy products

CDMO Viral Vector Services
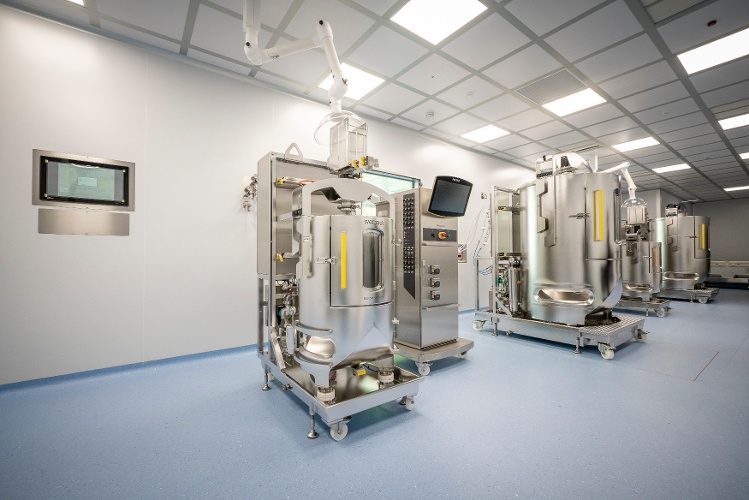
With our full-service viral vector offering, we can develop, manufacture, and optimize lenti (LVV), retro (RVV), and adeno-associated (AAV) viral vectors.
Extensive Experience Through Commercial
Benefiting from over 30 years of industry-leading expertise and four commercially approved products, AGC Biologics is at the forefront of viral vector clinical and commercial manufacturing capabilities with substantial GMP capacity, approved by regulatory authorities.

Fast & Scalable Vector Production
Our ready-to-use platform capabilities for AAV and LVV programs are built on cell factories and bioreactors using adherent and suspension processes. cGMP viral vector manufacturing for suspension-based processes provides up to 2,000 L. We also utilize iCELLis 500 bioreactor technology for adherent processes up to 750 L.

Full Lifecycle Development Systems
Our quality systems, manufacturing scales, and regulatory expertise allow us to meet both clinical and commercial viral vector demands. Moreover, our scale-down capabilities provide flexible and cost-effective solutions for process development and pre-clinical studies.
Viral Vector Manufacturing Track Record
0
Years of experience
0
Viral vector commercial approvals
+0
Manufactured viral vector GMP batches
End-to-End Viral Vector Services
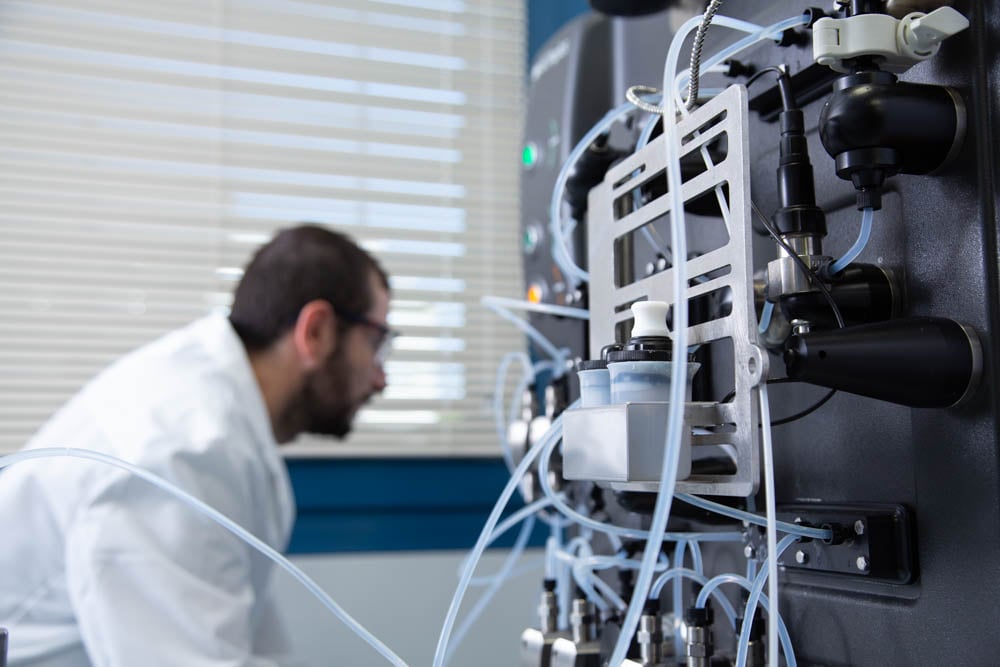
AGC Biologics scientists have three decades of viral vector-based gene therapy expertise and specialize in adeno-associated viral vectors (AAV), lentiviral vectors (LVV), and retroviral vectors (RVV). Our team has supported eight commercial approvals.
Our ready-to-use platform capabilities for AAV and LVV programs are built on cell factories and bioreactors using adherent and suspension processes, designed entirely in-house. Our scale-down capabilities provide flexible and cost-effective solutions for process development and pre-clinical studies.
Our quality systems, manufacturing scales, and regulatory expertise allow us to meet both clinical and commercial viral vector demands.
Got a project? Let's talk.
Our vector platforms that cut your timeline in half
End-to-end viral vector offerings give developers plug-and-play templates for the entire development and manufacturing process
Listen to Expert Interviews from Our Cell & Gene Center of Excellence in Milan

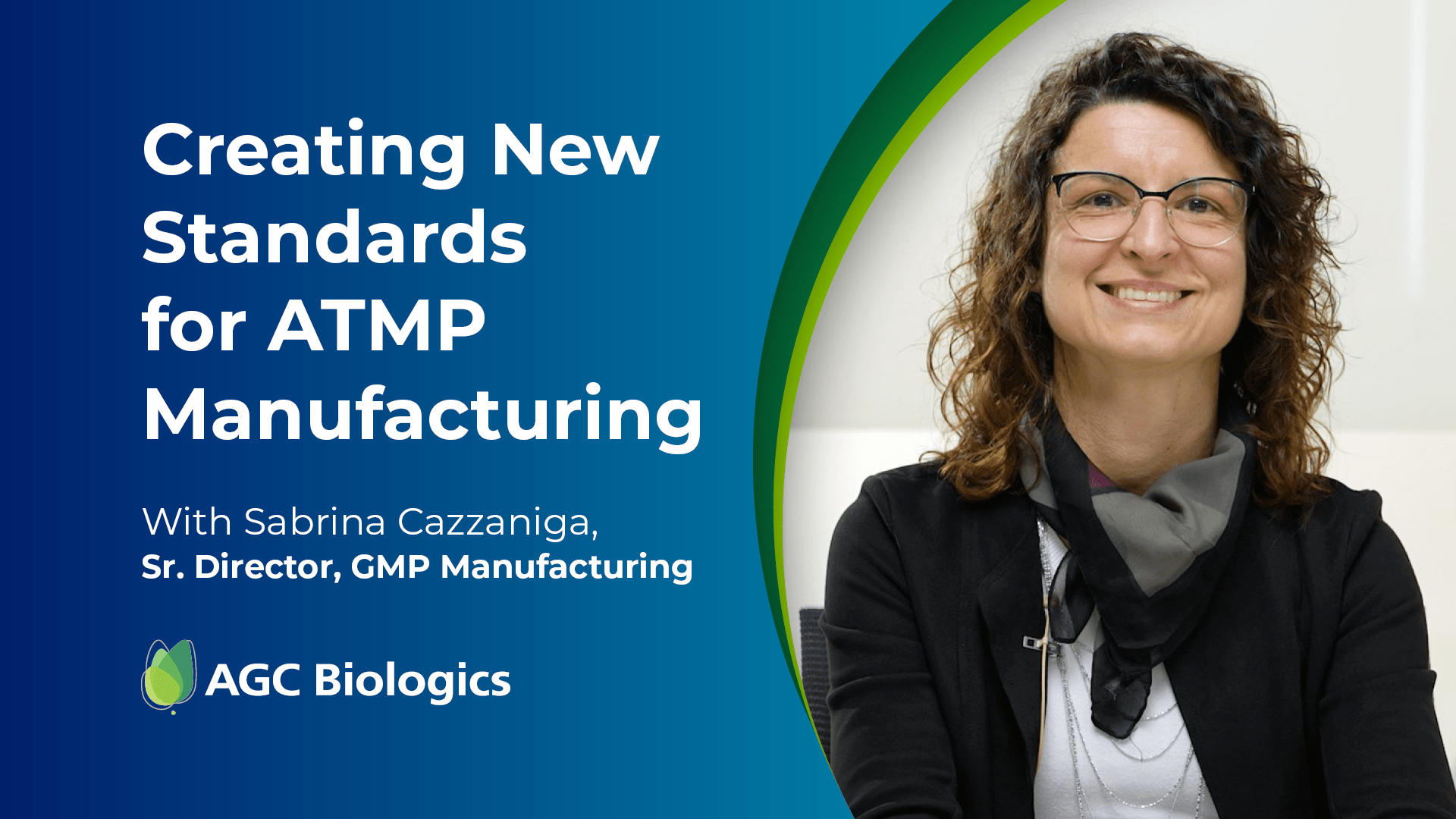
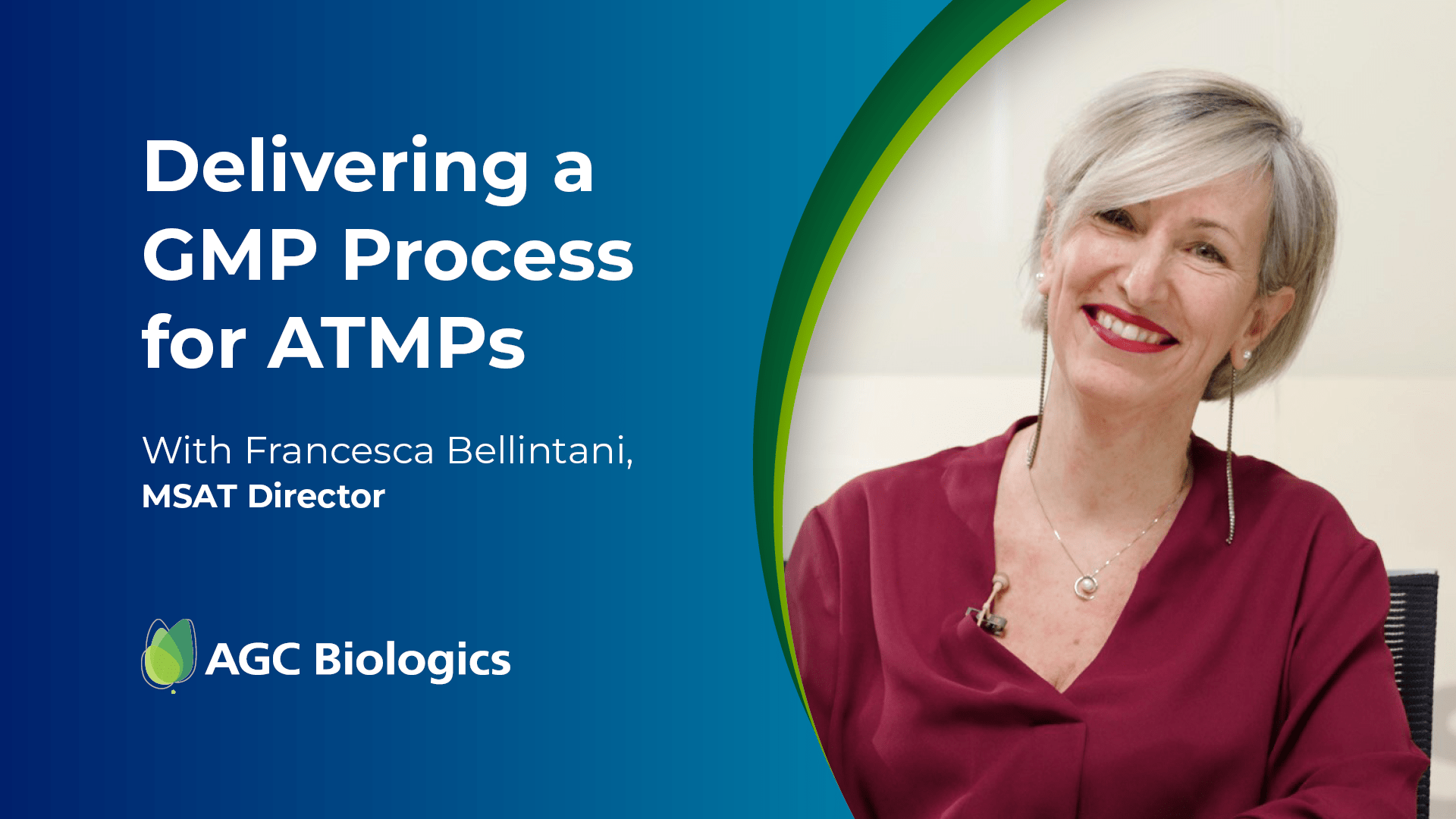
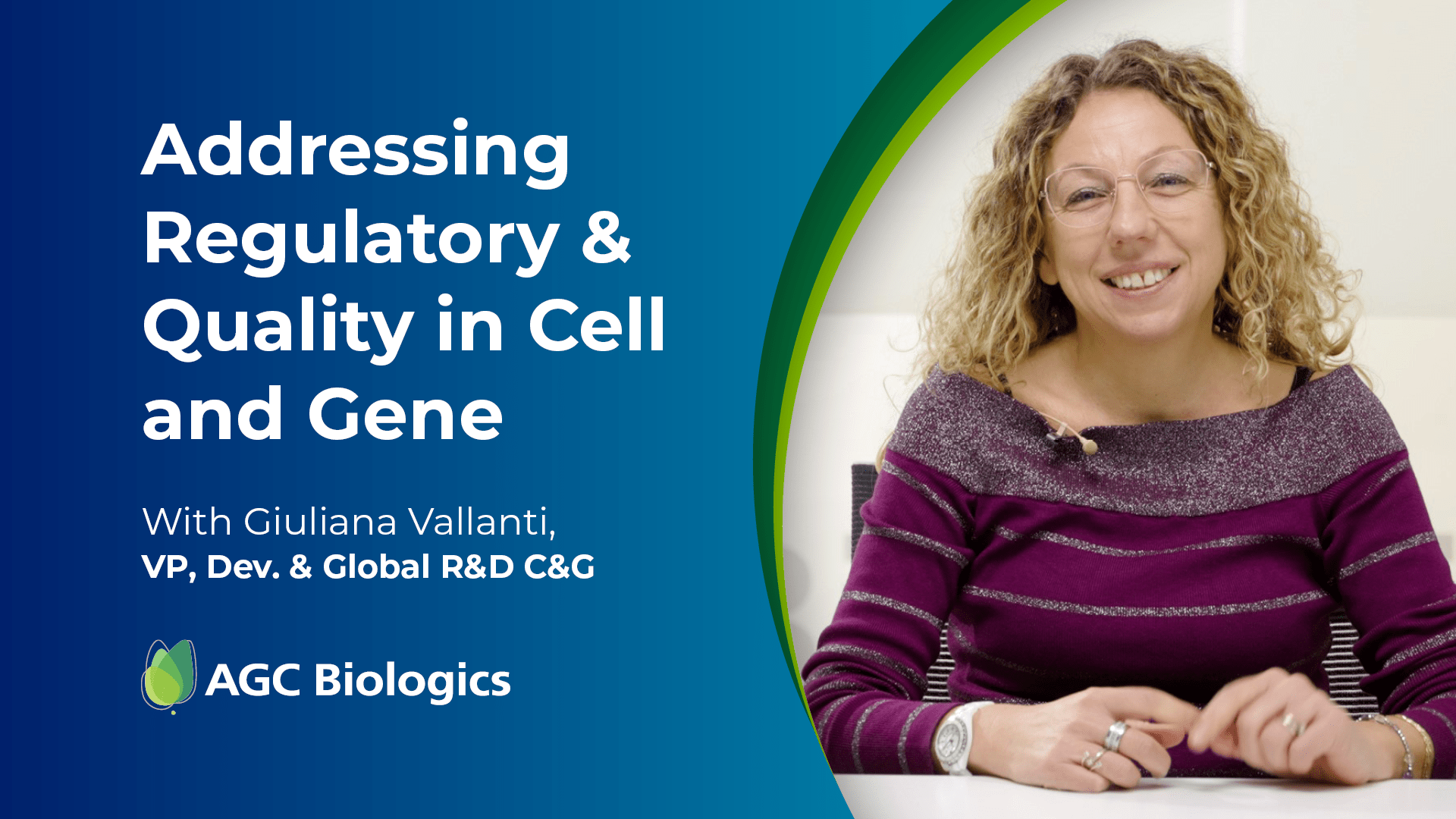
Click and drag video thumbnails to see more content.
Viral Vector Development from Clinical to Commercial

Adhesion and Suspension
Small, medium, and large-scale equipment for both adherent and suspension processes.

Plug-and-Play Platform
Plug-and-play platform for large-scale AAV and LVV manufacturing that can be adapted to virtually any program.
Optimizing Large-Scale AAV Manufacturing
Learn about independent, innovative processes for producing high-quality, high-productivity AAVs.
A Tailored Approach to Viral Vector Production
A custom platform offers scalable, off-the-shelf manufacturing to accelerate your timeline to GMP and save costs.
Download