Microbial
Fermentation Manufacturing
We offer extensive development and manufacturing services using microbial expression biologics systems

CDMO Microbial Services
Our best-in-class cell line and process development services offer a comprehensive range of capabilities to meet your specific product needs.
Microbial Manufacturing and Production
We have produced 100+ products using microbial-expressed proteins and multiple modes of expression - including secretion (yeast), periplasmic secretion, soluble intracellular expression, and inclusion bodies. The AGC Biologics network has produced seven commercial products using microbial systems.

Microbial Expression Systems
Our microbial services range from cell-line development and process development to clinical and commercial manufacturing. Our expertise includes experience with E. coli, Pichia pastoris with or without methanol induction, Pseudomonas Fluorescens (PFEnex system), Bacillus (non-sporulating), L. casei, and more.
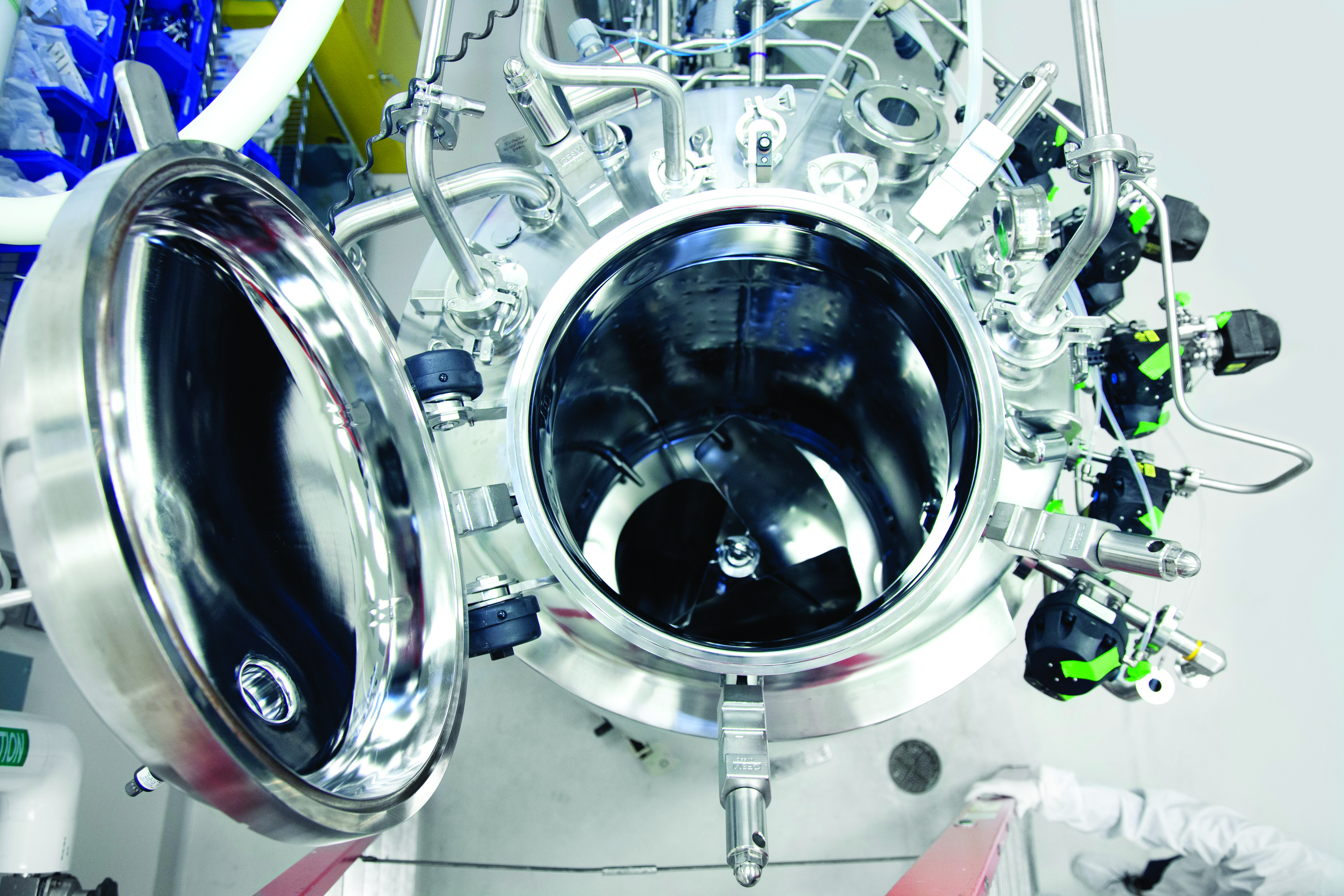
Microbial Manufacturing Scales
We offer flexible manufacturing scales up to 3,000 L and our cell banking services meet the standards of regulatory agencies around the world. We use standard industry systems, including batch and fed-batch methods, to produce microbial drug products.

Microbial Manufacturing Track Record
+0
Years of experience
0
Commercial microbial products
+0
Microbial processes & products produced
Creating Scalable Microbial Processes
Learn from our experts on how we overcome challenges in microbial process development when scaling up.
Microbial Drug Manufacturing Trends Driving the Future
Access this white paper to discover the changes affecting the microbial manufacturing industry.
DownloadFill out the form to access "Developing Reliable, Repeatable Microbial Processes at Any Scale"
Fill out the form to access "From Petri Dish to Profit: 5 Changes Driving the Future of Microbial Drug Manufacturing"
Microbial Fermentation Development
Our capabilities include methanol feeding, oxygen enrichment, continuous centrifugation, and high-pressure homogenization capabilities, ensuring the highest quality and efficiency throughout the manufacturing process. Our cGMP-compliant systems utilize stainless steel fermenters at various scales. Whether you require small-scale production or large-scale manufacturing, our facilities are tailored to deliver optimal results for your microbial-based products.
Got a project? Let's talk.
Special Offer
10,000 L GMP Production, Guaranteed Slots, & Set Pricing.*
*Terms and conditions apply. Limited-time offer until March 31, 2026.
Microbial Development from Clinical to Commercial

E. Coli and P. Pastoris
The ability to optimize media feeds and standard processes for high cell density E. coli and P. pastoris production.










